
Nuclear battery delivers 10x more energy
Russian researchers have developed a nuclear battery that delivers 10x more energy per gram than current button cell batteries. The battery has an operating life of a number of decades.
The nuclear battery uses nickel-63 as its source of radioactivity. This nickel isotope decays to stable copper by radiating electrons, and has a half life of 101 years. As an isotope, nickel-63 has the same chemical properties as stable nickel-58, but with a number of extra neutrons in the nucleus.
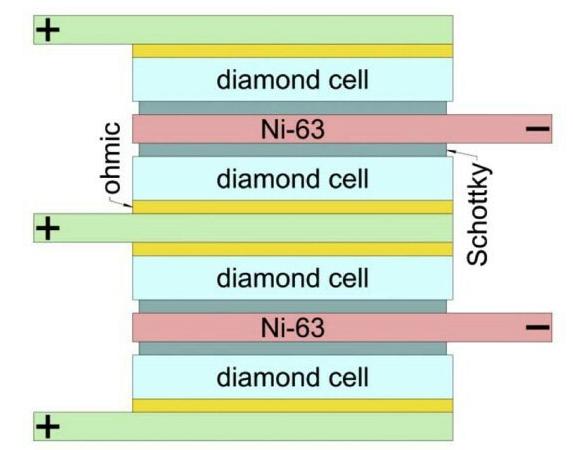
The key achievement of the researchers from the Technological Institute for Superhard and Novel Carbon Materials in Moscow is primarily the way in which they managed to construct a battery using nickel-63. They achieved an energy density that is ten times better than previous nickel batteries.
They built the battery using layers of nickel-63 measuring just 2 µm (1 µ=0.001 mm), sandwiched between two layers of specially produced synthetic diamonds of 10 µm each that act as diodes. These ensure that the electrons can only travel in one direction. In total, the battery comprises some hundred layers of nickel and diamond.
Microwatts
The amount of energy the battery can deliver is in the sphere of small numbers. At a scant 1 volt, it doesn't even deliver a microwatt of power. But because nickel-63 decays very slowly, the battery has an energy supply of 3300 mWh per gram, and that is ten times more than most Li-ion or silver-zinc button cell batteries.
The main benefit of a nuclear battery is its long operating life. That also explains why earlier versions were used in the seventies to power pacemakers. Although based on a different operating principle, these batteries are currently used in space vehicles, such as the Mars rovers. These batteries use the heat caused by the nuclear radiation to generate electricity via a thermocouple.
The University of Bristol is currently working on an alternative radioactive source, carbon-14, with a half life of 5700 years.
It's still not clear which applications these batteries would be used in. Because they use difficult-to-produce radioactive isotopes, they are more likely to be used in a specific niche than in all sorts of sensors currently being developed in the context of the Internet of Things.
If you found this article interesting, subscribe for free to our weekly newsletter!
Meer artikelen

Hoe gaat het met de energietransitie in Europa?

Martijn Otten: Met techniek het verschil maken

Martine Stam: Water is de rode draad in mijn leven

Kirsten Steinbusch: Iedereen kan iets betekenen in de transitie naar circulair
Nieuwste artikelen

Hoe gaat het met de energietransitie in Europa?

Martijn Otten: Met techniek het verschil maken




