Potassium improves perovskite-based solar cells
Solar cells made of perovskite can be made more stable by adding a small amount of potassium iodide. This has been demonstrated by material scientists from Delft and Cambridge. The potassium ions secure the material at a microscopic scale, so that it degrades more slowly. What's more, it also becomes more efficient thanks to the potassium.
More and more roofs are covered with solar panels, most of which are of the traditional type: crystalline silicon. Basically satisfactory, but scientists and engineers are working on a new generation of solar cells with greater levels of efficiency. After all, wouldn’t it be great to generate more electricity using the same amount of sunlight?
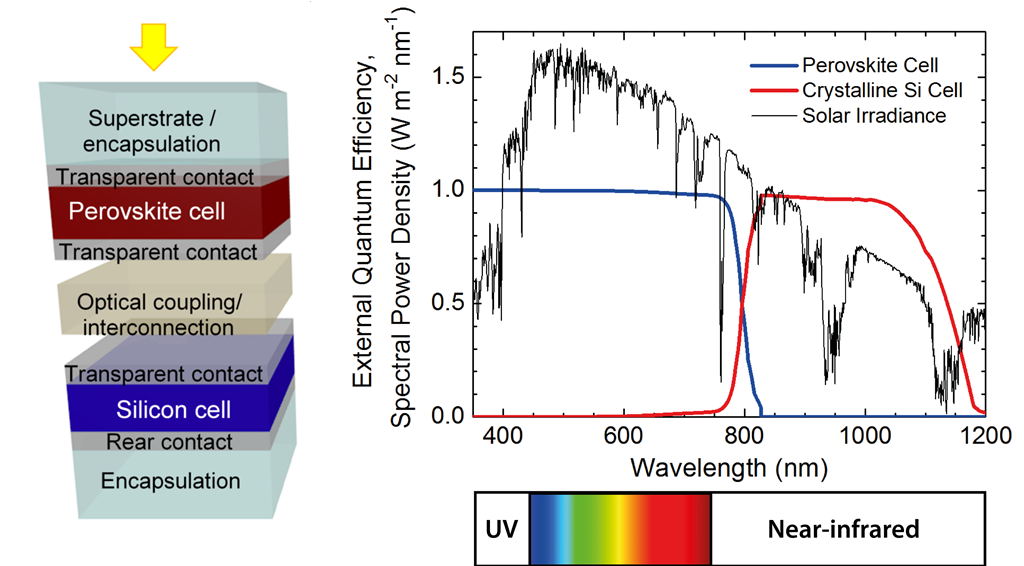
One of the main candidates for improved solar cells is the so-called tandem cell. This is a solar cell that basically comprises two separate types of solar cell stacked on top of one another. The first layer reached by the sunlight is a solar cell made of perovskite. This is a family of materials that is much easier to process than the silicon of traditional solar panels (read: ‘Very stable perovskite solar cell’). One additional advantage is that the properties of perovskite can be quite easily tuned, including the band gap, which determines the wavelengths at which the material absorbs light.
The smart thing about such tandem cells is that while the perovskite absorbs most of the visible light, turning it into power, the silicon cell then converts infrared light to electricity. The two materials complement each other by harvesting a different part of the spectrum of sunlight (see figure below).
STABILITY
Now perovskite-based solar cells, containing halides (iodides, bromides), suffer from problems with stability, as the material degrades too quickly. ‘Under the influence of the incoming sunlight, the halides in the perovskite start to move,’ explains university lecturer Tom Savenije from Delft University of Technology.
Motivated by this, one of his PhD students, Eline Hutter collaborated with Cambridge University to research ways of arresting the movement of the ions concerned. And they have now succeeded, as witnessed by a publication in Nature last week. Adding potassium iodide to the perovskite layer secures the halides, thus halting or retarding the degradation process.
'We tested various solar cells for a month. Over that period, the cells containing potassium iodide were significantly more stable than the cells without it.' No degradation at all was observed in cells with potassium iodide that had not been used, as stated in the paper. Cells illuminated for 300 hours on full power, retained 80% of their original efficiency at the end of the test. That is comparable to 30 days of sunlight for 10 hours a day. So there is a lot of work still to be done to secure the performance of solar cell material for longer periods.
ERRORS IN THE GRID
But there was a further problem with perovskite. Errors in the material's crystalline grid mean that electrons released by sunlight can't be extracted effectively to the circuit. They basically become trapped in the material and no longer contribute to the efficiency of the solar cell.
The potassium iodide was proven to have a positive effect on this as well, as it largely resolves the defects. ‘This means the electrons are more effectively extracted, which results in improved efficiency of the solar cells,’ says Hutter in a press release of Delft University of Technology.
CHARGE TRANSPORT
The Cambridge University researchers contributed most of the materials knowledge for this project. The researchers in Delft are specialised in measuring charge transport through the solar cell material. The test setup involves a bright laser pulse being aimed at the material, giving an idea of how the free charge moves through the cells.
Perovskite solar cells are made by spin coating the necessary materials from a solution. The potassium is applied to the solar cell by adding potassium iodide to this mix. This promises a simple, affordable and scalable adaptation of the existing production method for perovskite solar cells.
GAINS AT PRODUCTION
Savenije emphasises that the results at lab scale are looking positive, but the real gains will be seen once solar cell producers add potassium to their production process. In the Netherlands, Solliance is working on the above-mentioned tandem cells of perovskite and silicon. Solliance focuses on developing production techniques for flexible solar cells on foil. Savenije: ‘We have tested a number of different potassium concentrations between 5 and 40%, but it's quite possible that this can be improved. This fine-tuning is a key task of an institute or company and we have now been in contact with Solliance to this end.’
If you found this article interesting, subscribe for free to our weekly newsletter!
Opening photo: Solar cells of perovskite-tin from Oxford University (not related to the study described). Source: Oxford University